Although the sale of hydrochloric acid is limited and can be difficult to buy at retail, anyone can easily make it from polyvinyl chloride, one of the most common plastics. PVC is used to make wire sheaths, toys, and many household and industrial products.
For our purposes, PVC is needed in the form of chips.

Let's build a simple apparatus. There is water in the glass, over which the outlet from the tube is covered with a rubber “umbrella”. The snorkel and umbrella should not touch the water. The test tube must be completely dry on the outside (otherwise it will burst). The cotton wool is not installed tightly. Heat the chips.

Thermal decomposition of PVC releases gaseous HCL, which dissolves in water. In addition, if we are not dealing with pure PVC, but with plastics based on it (which is usually the case), then along with HCL all sorts of foul-smelling nasty things are released into the air, so the experiment should be carried out in appropriate conditions.

We continue the process until the chips turn black. It can be longer, but the HCL will not increase noticeably, and washing the tube will be problematic.

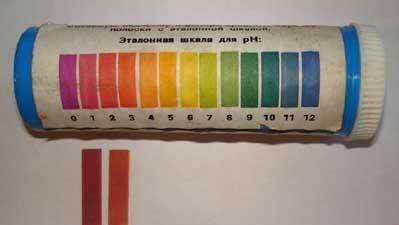
On the left is a test of a “purchased” 10% HCL solution, on the right is our “homemade” solution.Apparently, its concentration is about
5%.
The resulting hydrochloric acid oxidizes metals (especially zinc), reacts with alkalis, etc. - in general, it behaves as expected from hydrochloric acid.